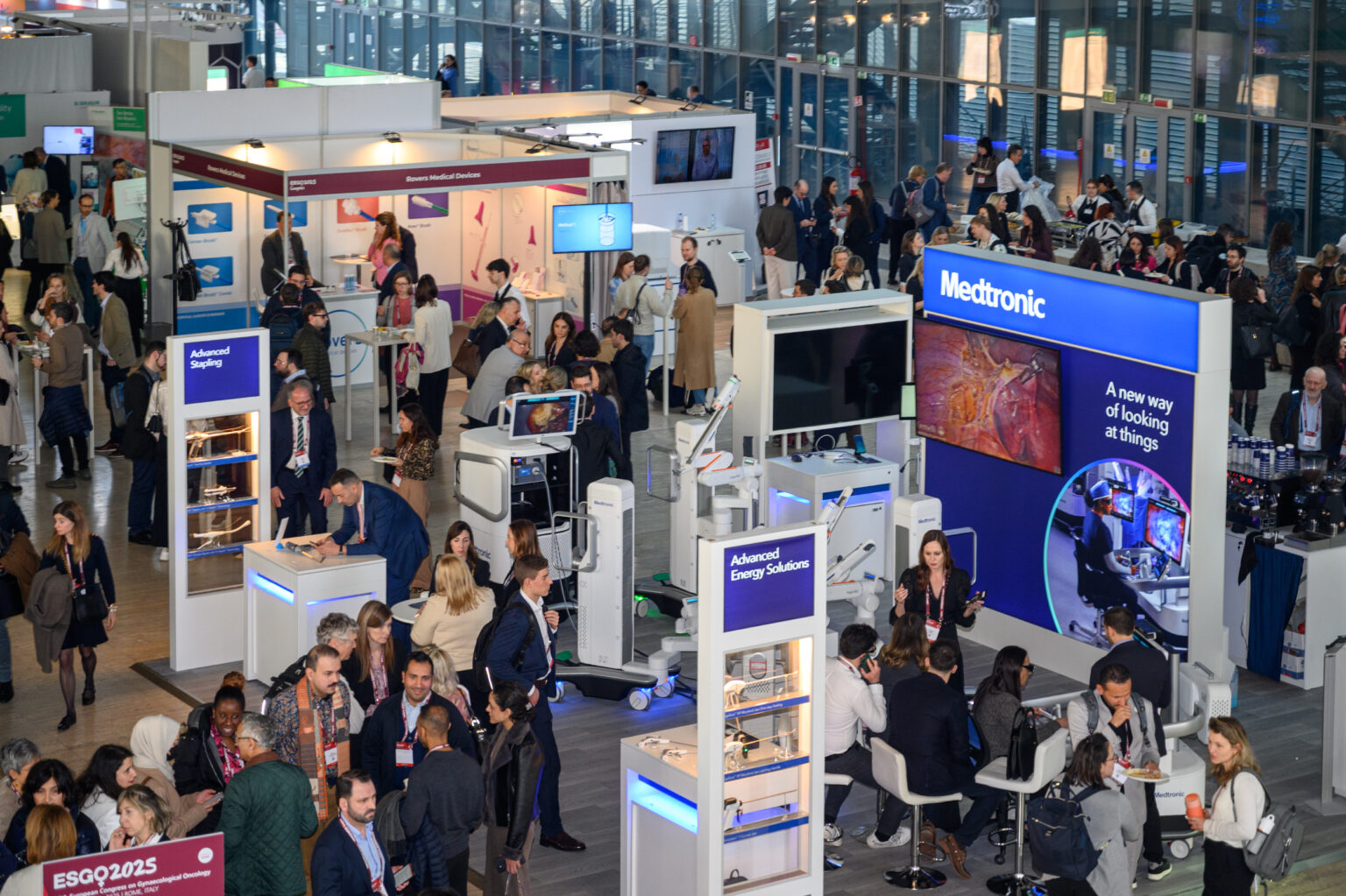
Sponsorship Benefits
ESGO 2026 is the landmark event in the field of gynaecological cancers, don't miss out on your opportunity to join us in Copenhagen to:
- Showcase your role in the field to an audience of over 3000 global participants
- Promote your products to world experts and professionals in gynaecological oncology, oncology and related professions
- Be associated with innovative educational opportunities connecting world experts and delegates
- Open up or build on new markets and networking opportunities with world leading influencers and decision makers
Ethical MedTech Application
 27th Congress of the European Society of Gynaecological Oncology has been submitted to Ethical MedTech.
27th Congress of the European Society of Gynaecological Oncology has been submitted to Ethical MedTech.
Event EMT-25-06358 is compliant.
For more details please check here.
We strongly encourage industry partners to join us at the 27th European Congress on Gynaecological Oncology for a unique opportunity to engage with thought leaders, researchers, clinicians, and decision-makers in the oncology field.
As the largest European and most comprehensive gynaecologic oncology event, attracting over 3000 participants annually, this congress provides an invaluable platform to showcase innovations, foster collaborations, and shape the future of cancer care.
Why should industry join ESGO 2026?
Showcase cutting-edge innovations
This congress offers industry leaders a chance to present and demonstrate their most advanced products, therapies, and technologies to a highly engaged audience of gyn-onc professionals. Whether it’s novel therapeutics, diagnostic tools, medical devices, or digital health solutions, your innovations will be front and center, helping to establish your company as a leader in the fight against cancer.
Collaborate with researchers and experts
The congress presents a prime opportunity to establish new partnerships and collaborations with academic institutions, hospitals, and leading researchers. Collaboration with experts can drive the next generation of cancer treatments and innovations, enhancing your company’s impact on patient outcomes worldwide.

Maximize brand visibility
The ESGO 2026 congress provides unmatched exposure, allowing industry partners to increase their visibility and enhance their brand reputation. Through sponsored sessions, workshops, or exhibition booths, you can demonstrate your commitment to advancing cancer research and treatment.

Contribute to the future of cancer care
By participating in this congress, industry partners have the opportunity to make a direct impact on the future of cancer care. Your involvement in this important event demonstrates your commitment to improving patient outcomes and advancing cancer research. Through collaboration, knowledge sharing, and innovation, we can drive the collective effort to revolutionize cancer treatment and ultimately save lives.

Access to a global network
This event will attract gynaecological oncology professionals from around the world, offering you the chance to connect with a truly international audience. The diverse attendee base includes gynaecologists, gynaecological and medical oncologists, radiation oncologists, researchers, healthcare institutions, and patient advocacy groups. This global network can help open new business opportunities, increase market reach, and build lasting relationships with partners across the gyn-onc community.
Any Questions?
To find out more about ESGO 2026 sponsorship and exhibition opportunities,
please contact: Zuzana Seps, Director of Congress and Educational Events
Tel: +420 725 537 730
Email: zuzana.seps@esgo.org
